NCS presents “Arthroscopic Transosseous Rotator Cuff Repair”, the first monograph about transosseous
Tomorrow “Arthroscopic Transosseous Rotator Cuff Repair Tips and tricks” will be presented at the ICOT Institute (Surgery, Orthopaedy, Traumatology Institute) in Latina. Matteo Mantovani, CEO of NCS will talk about the arthroscopic surgical technique with the NCS Lab | Medical Devices Taylor Stitcher and about how this procedure improves the process of reconstruction of the rotator cuff. The procedure with Taylor Stitcher also guarantees several benefits for patients but also minimizes costs for operating rooms:

“This book covers all aspect of the transosseous approach, starting from foundation of biological science and progressing through postoperative rehabilitation. Along the way, the chapters discuss biomechanical factors and technologies in the marketplace, tracing the evolution over time, proposing solution and offering a view on the future. All of the material is supported by an impressive reference collection, which provides infinite possibilities to deepen one’s knowledge on the subject”
At the presentation, along with Matteo Mantovani, there will also be other authors:
- Claudio Chillemi – Author – Orthopaedic Aid at ICOT
- Alessandro Castagna – Author – Director of the Shoulder Operational Unit at the Humanitas Institute, Milan
- Marcello Osimani – Author – Radiology Operational Unit Polo Pontino
After the presentation, the audience will have the chance to attend a live surgery held by Claudio Chillemi and Alex Castagna.

Read an excerpt of the book here:
LOOK INSIDE: https://www.amazon.com/Arthroscopic-Transosseous-Rotator-Cuff-Repair/dp/3319761528/ref=mt_hardcover?_encoding=UTF8&me=&qid=1535559342
Further information about event here:
NCS | Taylor Stitcher:
https://ncs-company.com/case-history/medical-devices/sport-medicine/taylor-stitcher/
NCS goes to Boston for the event “Transosseous Academy 2018”
“Transosseous Academy”, the first international convention and the second annual event, has just finished in Boston. NCS was main sponsor.
“Transosseous Academy”, the first international convention and the second annual event, has just finished in Boston. NCS was main sponsor. “Transosseous Academy” is an independent and non-profit association born in order to promote and support scientific research, innovation and foster knowledge sharing on the transosseous surgical technique.
The mission of the association is to put together clinicians, engineers and scientists to create an interactive environment in which propose and carry out research leading to more and more positive results for patients.
Transosseous Academy combines the engineering sphere with the clinical and medical sphere in a brand-new field oriented towards science and the sharing of results. TA’s primary objective is to collect clinical data in order to highlight and support the surgical treatments studied.
NCS | Medical Devices supports the project “Transosseous Academy” realised in Milan during the first meeting in 2017 and in the first international event held in Boston last 10th of November. NCS has always been active in the processes of design and production of implantable biomedical devices intended and dedicated to arthroscopic techniques which became even more effective. For this reason, NCS sustains the mission of Transosseous Academy.
An international faculty held speeches, lectio magistralis and re-live. The various interventions covered all the aspects of transosseous from biomechanics to biology, from the several techniques available today to a new way of collecting in an objective way clinical data leading to clinical results. An exceptional faculty able to elevate scientific content and maintain an interactive and stimulating environment.
NCS and Transosseous Academy would like to thank Dr Alessandro Castagna, chairman and former supporter of TA all the members of the faculty (mentioned below). A sincere thanks to Dr Snyder who shared his unbelievable experience and carrier full of success and innovation.

Faculty:
Stephen Snyder, MD – USA
August Mazzocca, MD – USA
Claudio Chillemi, MD-ITA
Cory Edgar, MD – USA
Myriam Capasso, MD – VE
Andrea Pellegrini, MD – ITA
Jean Kany, MD – FRA
Sumant Krishnan, MD – USA
Uma Srikumaran, MD – USA
Matteo Mantovani, Eng.-ITA
UNI CEI EN ISO/IEC 17025:2005 Certification
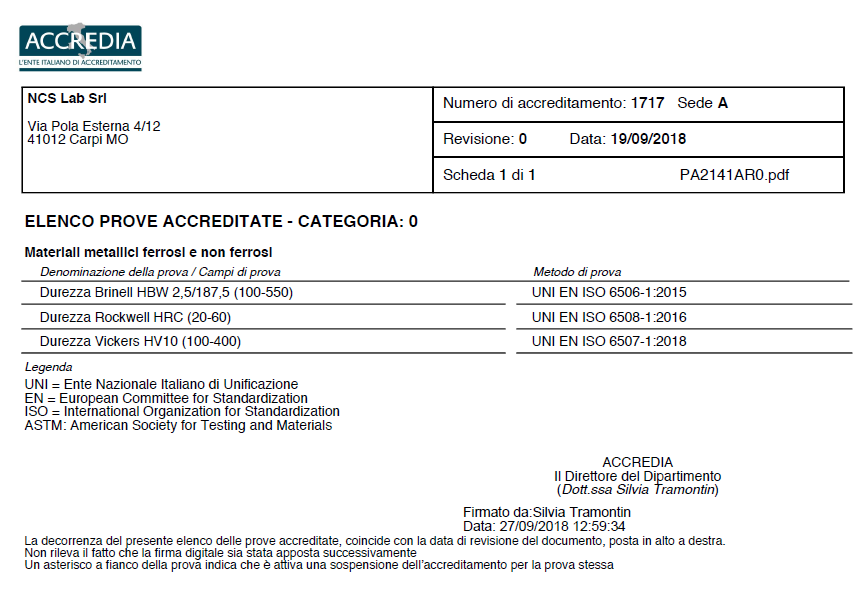
NCS Lab srl has obtained ACCREDIA accreditation according to the UNI CEI EN ISO/IEC 17025:2005 standard.
We are pleased to announce the certification of the Accredited Laboratory LAB No. 1717L of September 19, 2018: "General requirements for the competence of testing and calibration laboratories" for the following tests:
- Hardness Brinell HBW 2,5/187,5 (100-550)
- Hardness Rockwell HRC (20-60)
- Hardness Vickers HV10 (100-400)
Being in compliance with the requirements of ISO/IEC 17025:2005 means to possess both the technical competence required to carry out the analyses and a quality management system, which is essential to guarantee the correctness of the analytical data and the traceability of the measurements.
As accredited laboratory, NCS is able to provide reliable, credible and nationally and internationally accepted declarations of conformity and guarantees:
- Impartiality
- Independence
- Correctness
- Competence
The UNI CEI EN ISO/IEC 17025:2005 standard is international and is accepted in all countries of the world.
The list of tests approved is available at www.accredia.it
ACCREDIA assesses the technical competence and professional suitability of the operators of the Testing Laboratories, verifying their compliance with mandatory rules and voluntary standards, to ensure the value and credibility of certifications, inspections, tests and calibrations.
Accreditation ensures that test and inspection reports and certifications (system, product and personnel) bearing the ACCREDIA mark are issued in compliance with the most stringent international requirements for conformity assessment, and behind constant and rigorous surveillance of the behaviour of testing laboratories.
Test list
 Certificate
Certificate



